Molecular Dynamics Simulation of Monolayer Fullerene Membranes for Desalination
-
摘要: 海水淡化是最有希望解决全球淡水资源短缺的有效方案之一,纳米技术的进步推动了各类用于水净化的纳米多孔膜的发展. 理论和实验研究发现了纳米多孔石墨烯的超高水透过和盐离子拒绝率. 然而精确创建、控制纳米级孔隙的大小和分布的操作难度极大地限制了纳米膜材料的实际化应用. 通过分子动力学模拟发现具有均匀有序纳米孔排列准四边形结构(quasi-tetragonal phase, qTP)的单层富勒烯(C60)薄膜在海水淡化方面的巨大潜力,在保证100%阻盐率的同时,与传统聚合物过滤膜相比,单层富勒烯薄膜展示出卓越的透水性. 从原子尺度系统地研究了单层富勒烯薄膜结构的筛分机制,发现钠离子、氯离子与水分子相比,在穿膜运输过程中有大的能量障碍. 结果表明,单层富勒烯薄膜是一种很有优势的海水淡化膜.Abstract: Seawater desalination is one of the most promising solutions to fresh water shortage all over the world. The rapid development of nanotechnology led to the boom of nanoporous membranes for water purification. Recent theoretical and experimental studies reported ultra-high water permeability and salt rejection in nanoporous monolayer graphene. However, the difficulty of precisely creating nanometer-scale pores and controlling their distribution greatly limits its industrial application. Through molecular dynamics (MD) simulation, the monolayer quasi-tetragonal phase fullerene (qTPC60) was found to have tremendous potential as ultra-permeable membranes for desalination due to their unform pore distribution. The monolayer fullerene membranes exhibit high water permeability compared to conventional polymer filtration membranes. The work offers insights into the molecular mechanism of sieving, and the MD simulations show that Na+ and Cl- ions have large energy barriers. This 2D monolayer carbon material with unique structure exhibits great potential in seawater desalination.
-
Key words:
- seawater desalination /
- monolayer fullerene /
- water permeability /
- ion rejection /
- molecular dynamics
-
表 1 离子、水分子(SPC/E)和C60的LJ参数以及电荷信息
Table 1. The LJ parameters and partial charges for ions, water molecules (SPC/E), and carbon atoms of C60 and graphene
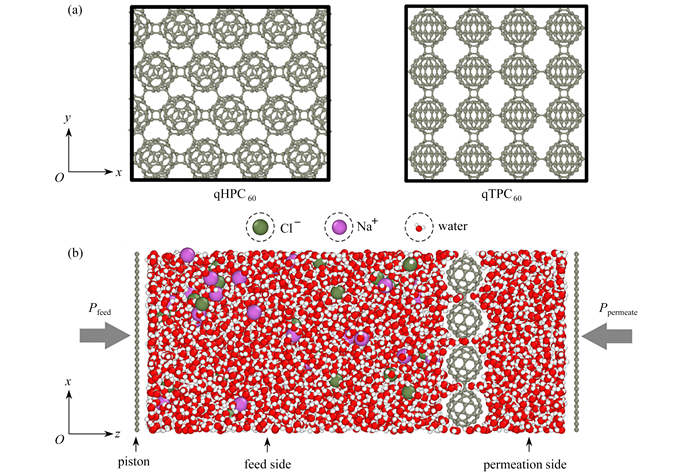
site σ/nm ε/(kcal/mol) q/e ion Na+ 0.333 0.002 772 1.0 Cl- 0.442 0.117 8 -1.0 water H 0 0 0.423 8 O 0.317 0.153 5 -0.847 6 C60 C 0.340 0.086 0 graphene C 0.340 0.086 0 表 2 第一、第二水化壳层在体相水、qTPC60内部的配位数以及相应脱水数目
Table 2. Coordination numbers in the 1st and 2nd hydration shells and reduced numbers
Bulk N qTPC60 N reduced number Nr Nc1 5.6 2.0 3.6 Nc2 17.1 3.2 13.9 -
[1] ANDREEVA D V, TRUSHIN M, NIKITINA A. Two-dimensional adaptive membranes with programmable water and ionicchannels[J]. Nature Nanotechnology, 2021, 16(2): 174-180. doi: 10.1038/s41565-020-00795-y [2] WERBER J R, OSUJI C O, ELIMELECH M. Materials for next-generation desalination and water purification membranes[J]. Nature Reviews Materials, 2016, 1(5): 16018. doi: 10.1038/natrevmats.2016.18 [3] ELIMELECH M, PHILLIP W A. The future of seawater desalination: energy, technology, and the environment[J]. Science, 2011, 333(6034): 712-717. [4] GEISE G M, PARK H B, SAGLE A C. Water permeability and water/salt selectivity tradeoff in polymers for desalination[J]. Journal of Membrane Science, 2011, 369(1/2): 130-138. [5] CORRY B. Water and ion transport through functionalised carbon nanotubes: implications for desalination technology[J]. Energy Environment Science, 2011, 4(3): 751-759. doi: 10.1039/c0ee00481b [6] SURWADE S P, SMIRNOV S N, VLASSIOUK I V. Graphynes for water desalination and gas separation[J]. Nature Nanotechnology, 2015, 10(5): 459-464. doi: 10.1038/nnano.2015.37 [7] QIU H, XUE M M, SHEN C. Graphynes for water desalination and gas separation[J]. Advanced Materials, 2019, 31(42): e1803772. doi: 10.1002/adma.201803772 [8] YANG Y B, YANG X D, LIANG L. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration[J]. Science, 2019, 364(6445): 1057. doi: 10.1126/science.aau5321 [9] O'HERN S C, BOUTILIER M S H, IDROBO J C. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes[J]. Nano Letter, 2014, 14(3): 1234-1241. doi: 10.1021/nl404118f [10] COHEN-TANUGI D, GROSSMAN J C. Water desalination across nanoporous graphene[J]. Nano Letter, 2012, 12(7): 3602-3608. doi: 10.1021/nl3012853 [11] KONATHAM D, YU J, HO T A, et al. Simulation insights for graphene-based water desalination membranes[J]. Langmuir, 2013, 29(38): 11884-11897. doi: 10.1021/la4018695 [12] SINT K, WANG B Y, KRAL P. Selective ion passage through functionalized graphene nanopores[J]. Journal of the American Chemical Society, 2008, 130(49): 16448-16449. doi: 10.1021/ja804409f [13] FENG J D, GRAF M, LIU K. Single-layer MoS2 nanopores as nanopower generators[J]. Nature, 2016, 536(7615): 197-200. doi: 10.1038/nature18593 [14] CAO Z L, LIU V, FARIMANI A B. Water desalination with two-dimensional metal-organic framework membranes[J]. Nano Letter, 2019, 19(12): 8638-8643. doi: 10.1021/acs.nanolett.9b03225 [15] HOU L, CUI X, GUAN B, et al. Synthesis of a monolayer fullerene network[J]. Nature, 2022, 606(7914): 507-510. doi: 10.1038/s41586-022-04771-5 [16] PENG B. Monolayer fullerene networks as photocatalysts for overall water splitting[J]. Journal of the American Chemical Society, 2022, 144(43): 19921-19931. doi: 10.1021/jacs.2c08054 [17] PENG B. Stability and strength of monolayer polymeric C60[J]. Nano Letter, 2023, 23(2): 652-658. doi: 10.1021/acs.nanolett.2c04497 [18] YU L F, XU J Y, PENG B, et al. Anisotropic optical, mechanical, and thermoelectric properties of two-dimensional fullerene networks[J]. Journal of Physical Chemistry Letters, 2022, 13(50): 11622-11629. doi: 10.1021/acs.jpclett.2c02702 [19] YING P H, DONG H K, LIANG T, et al. Atomistic insights into the mechanical anisotropy and fragility of monolayer fullerene networks using quantum mechanical calculations and machine-learning molecular dynamics simulations[J]. Extreme Mechanics Letters, 2023, 58: 101929. doi: 10.1016/j.eml.2022.101929 [20] KAYAL A, CHANDRA A. Exploring the structure and dynamics of nano-confined water molecules using molecular dynamics simulations[J]. Molecular Simulation, 2015, 41(5/6): 463-470. [21] SHAO Q, ZHOU J, LU L, et al. Anomalous hydration shell order of Na+ and K+ inside carbon nanotubes[J]. Nano Letter, 2009, 9(3): 989-994. doi: 10.1021/nl803044k [22] ZHANG X, WEI M, XU F, et al. Pressure-dependent ion rejection in nanopores[J]. Journal of Physical Chemistry C, 2020, 124(37): 20498-20505. doi: 10.1021/acs.jpcc.0c03641 [23] ZHU F Q, TAJKHORSHID E, SCHULTEN K. Pressure-induced water transport in membrane channels studied by molecular dynamics[J]. Biophysical Journal, 2002, 83(1): 154-160. doi: 10.1016/S0006-3495(02)75157-6 [24] 齐进, 吴锤结. 可压缩Navier-Stokes方程的时空耦合优化低维动力系统建模方法[J]. 应用数学和力学, 2022, 43(10): 1053-1085. doi: 10.21656/1000-0887.430220QI Jin, WU Chuijie. Construction of spatiotemporal-coupling optimal low-dimensional dynamical systems for compressible Navier-Stokes equations[J]. Applied Mathematics and Mechanics, 2022, 43(10): 1053-1085. (in Chinese) doi: 10.21656/1000-0887.430220 [25] LI L X, DONG J H, NENOFF T M. Desalination by reverse osmosis using MFI zeolite membranes[J]. Journal of Membrane Science, 2004, 243(1/2): 401-404. [26] COHEN-TANUGI D, GROSSMAN J C. Water permeability of nanoporous graphene at realistic pressures for reverse osmosis desalination[J]. Journal of Chemical Physics, 2014, 141(7): 074704. doi: 10.1063/1.4892638 [27] GUILLEN G, HOEK E M. Modeling the impacts of feed spacer geometry on reverse osmosis and nanofiltration processes[J]. Chemical Engineering Journal, 2009, 149(1/3): 221. [28] HEIRANIAN M, FARIMANI A B, ALURU N R. Water desalination with a single-layer MoS2 nanopore[J]. Nature Communications, 2015, 6: 8616. doi: 10.1038/ncomms9616 [29] HUMMER G, RASAIAH J C, NOWORYTA J P. Water conduction through the hydrophobic channel of a carbon nanotube[J]. Nature, 2001, 414(6860): 188-190. doi: 10.1038/35102535 [30] 曹玉玲, 何强胜, 刘闯. 考虑页岩塑性变形的水力裂缝与天然裂缝相交模拟研究[J]. 应用数学和力学, 2023, 44(6): 679-693. doi: 10.21656/1000-0887.430300CAO Yuling, HE Qiangsheng, LIU Chuang. Numerical simulation of hydraulic fractures intersecting natural fractures in shale with plastic deformation[J]. Applied Mathematics and Mechanics, 2023, 44(6): 679-693. (in Chinese) doi: 10.21656/1000-0887.430300 [31] HE Z J, ZHOU J, LU X, et al. Ice-like water structure in carbon nanotube(8, 8) induces cationic hydration enhancement[J]. Journal of Physical Chemistry C, 2013, 117(21): 11412-11420. doi: 10.1021/jp4025206 [32] SUK M E, ALURU N R. Water transport through ultrathin graphene[J]. Journal of Physical Chemistry Letters, 2010, 1(10): 1590-1594. doi: 10.1021/jz100240r -




 下载:
下载:








 渝公网安备50010802005915号
渝公网安备50010802005915号